Experiment
OBJECT: -
To determine the strength of a given unknown solution of HCl by titrating it against with the help of a known solution of NaOH using phenolphthalein indicator.
REFERENCE
1. Parle A., " Pharmaceutical Chemistry 1 Laboratory Manual", CBS Publishers and Distributors Pvt. Ltd., Ed Ist, 2008, pp 59.
REQUIREMENTS: -
Chemical required: NaOH Solution, HCl solution, phenolphthalein indicator
Glassware required: - burette, conical flask, and beaker, dropper.
PROCEDURE:-
(a) Take a burette and wash it with distilled water. (b) Rinse and fill the solution HCl N/10 with the help of a funnel and set the initial burette reading as zero. Clamp it vertically to the burette stand.
(c) Rinse the pipette with water and then with the given NaOH solution.
(d) Pipette out 10ml of given NaOH (N/10) solution into a conical flask and add one or two
drops of methyl orange.
(e) Titrate it against the HCl (N/10) solution taken in the burette till the color of the solution in
the conical flask changes from a yellowish color to pink color
(f) Note down the final burette reading.
(g) Repeat the titration until concordant values are obtained.
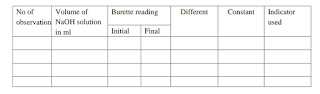
OBSERVATION:
Calculation:
N1V1=N2V2
RESULT:-
The strength of given unknown solution oh HCl is



.png)

0 Comments