Experiment
Synthesis of Tolbutamide
Object:
To prepare and submit tolbutamide from p-toluene sulfonamide and calculate its percentage yield.
Principle:
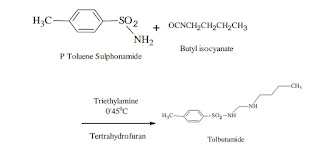
The synthesis of tolbutamide involves addition reaction of p-toluene sulfonamide and butyl isocyanate in the presence of triethylamine and tetrahydrofuran.
Reaction:
Chemical Required:
p-Toluene Sulfonamide – 1 g
Butyl isocyanate – 1 g
Triethylamine – 1.2 ml
Tetrahydrofuran – 10 ml
Glasswares:
RBF, pippet,beaker,funnel, glass rod etc
Procedure:
1. About N-butyl isocyanate (1m mol) and triethylamine (1.2m mol) in around bottom flask containing 10 ml of tetrahydrofuran, kept in an ice bath.
2. To the above mixture P-toluene sulfonamide (1m mol) was added drop wise at 0℃.
3. After completing the addition, the temperature was suddenly raised to 35-45℃ and stirred for 3-4 Hrs.
4. Then the solution was filtered. The product was separated and dried.
5. Then it was recrystalised by using Diethyl ether.
Melting Point: 128℃ to 129℃
Use:
It is aAnti-diabetic drug.
Observation:
Theoretical Yield:______mg
Practical Yield:_______mg
Percentage Yield:____%
Result:
The percentage of tolbutamide was found to be___%.
------------*****---------------
What is the use of tolbutamide.
Synthesis reaction of tolbutamide.
Tolbutamide experiment.



.png)

0 Comments